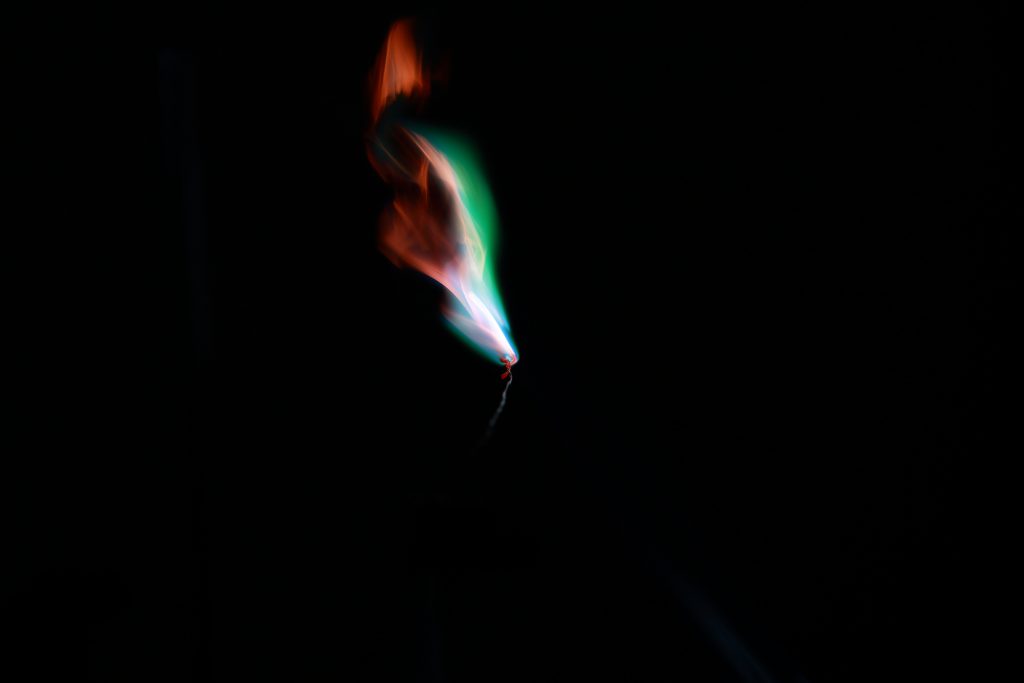


Title: The fantasy of copper chloride
The flame reaction is mainly based on the fact that some metals or their volatile compounds will show different colors when burned in the colorless flame. In the flame reaction experiment, different metals or their compounds emit a variety of different wavelengths of light when burned. In the visible light range perceived by the naked eye, the color presented is different due to the different wavelengths of the different light. In this photo, I used copper chloride to do this flame reaction. Although copper ion shows blue color during the flame reaction since tetrachloride copper complex formed by the combination of chloride ion and copper ion, which colors are yellow. Within the optical principle, when yellow meets blue, it will turn into green when we seeing this. So, the flame shows a beautiful green color with some of the red colors for burning the iron wire and a copper ion itself which turns out to be blue.



Title: Flaming wave
Butane is a colorless, liquefiable, and flammable gas at normal temperature and pressure. Often used as fuel, solvent, refrigerant, and raw material of organic synthesis. The burning process can be placed on hands is because butane is in bubbles, the butane gas tank is used to pass butane into the water to form bubbles. The heat had burned out before it reached the skin, with a layer of water was pasted on the hand and it’s touching the inner flame which has the lowest temperature in the fire. Whereas the hand can feel the heat of burning, it won’t reach the extent of burns because of the speed of the fire and the affection of water.












